
Discogel
What is Discogel?
Discogel is the trade name of Radiopaque Gelified Ethanol or RGE. It is manufactured by GELSCOM (France) and belongs to the class III medical device according to the Annex VIII classification of the European regulation 2017/745. Discogel has been on the market since 2007


Discogel is a sterile implantable medical device based on jellified ethanol in which an opaque agent in X-rays, the tungsten was added.
Discogel allows the treatment of herniated discs by decompression. This hospital product meets the requirements of patients and of practitioners wanting to achieve simple and efficient care which furthermore registers in the current pharmaco-economic mind of the health authorities.
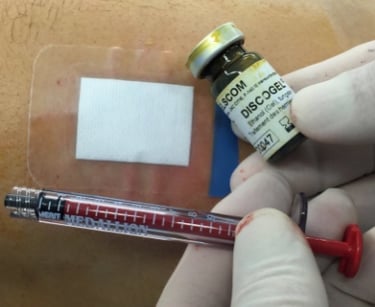
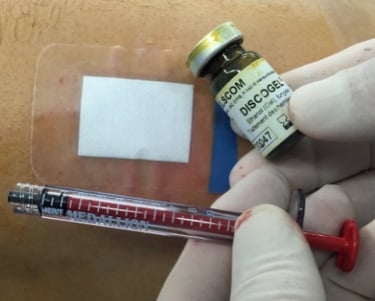
Discogel is introduced to the practitioner in a secondary packaging including a sterile vial containing the Discogel, an implant card dedicated to treated patients, an instruction for use, and a DATAMATRIX labeling to ease its traceability.
The treatment of the herniated disc by Discogel consists of injecting this radiopaque gel under fluoroscopic control in the intervertebral disc.
Official and updated information in PDF can be downloaded from the European database of medical devices. You will find a multilingual leaflet and a summary of safety and clinical performance established according to Article 32 of European Regulation 2017/745. You also can ask Smart Health Solutions Ltd. for any question related to Discogel.
For more information, please see the following pages:
